Targeting Cancer with a Triple Threat
A conversation with Professor Jeremiah A. Johnson on his development of a novel drug delivery system: bottlebrush prodrugs.
We often marvel at the wonders of modern medicine—the miracle cures, lifesaving vaccines, and targeted treatments. But beneath these advancements lies an unsung hero that makes it all possible: drug delivery systems. Without these carefully designed vehicles, even the most potent medicines fail to reach their full potential.
How drug delivery became essential
A couple decades ago, small molecules dominated the pharmaceutical landscape. These drugs, like aspirin or statins, relied on straightforward chemical and physical principles to enter the body and work their magic. Efforts at the time primarily focused on tweaking the molecules themselves: improving solubility, controlling release, or optimizing absorption.
But as treatments advanced, so did the challenges. Enter biologics: large, complex molecules like proteins, peptides, and nucleic acids. These groundbreaking therapies opened doors to previously untreatable conditions, but they came with significant hurdles. Proteins and peptides, for example, degrade easily in the body, while nucleic acids need to enter cells to work. A simple pill wouldn’t cut it anymore.
Suddenly, drug delivery systems—whether in the form of nanoparticles, liposomes, or hydrogels—weren’t just optional. They were indispensable. The modern era of medicine relies on these innovations to transform fragile molecules into effective therapies.
COVID-19 and the revolution in nanoparticles
The importance of drug delivery came into sharp focus during the COVID-19 pandemic. The much-lauded mRNA vaccines by Pfizer–BioNTech and Moderna were not just medical breakthroughs; they were also feats of engineering.
The star players? Lipid nanoparticles (LNPs).
These microscopic particles protected the mRNA from being destroyed by the body before it could reach its target. They also allowed the mRNA to slip inside cells, where it could instruct the body to produce its immune-boosting proteins. Without LNPs, the promise of mRNA-based therapies might still be a distant dream.
Why are nanoparticles so effective?
Protection from degradation. Molecules like mRNA are delicate and break down quickly in the body. Nanoparticles provide a shield, ensuring they stay intact long enough to work.
Controlled release. Injecting a drug directly can cause a sudden spike in concentration, potentially leading to side effects. Nanoparticles allow for a slow, steady release, reducing toxicity.
Cellular targeting. Nanoparticles are small enough to infiltrate cells, making them ideal for therapies that need to act inside the body’s building blocks.
Still, challenges remain. The mRNA vaccines needed ultra-cold storage—an obstacle in regions without cold-chain infrastructure. The vaccine was shipped at –70° Celsius and is usable from a refrigerator for about 10 weeks. Having to keep the vaccine in such cold temperatures was not convenient and meant that the vaccine could be inaccessible to areas where cold chain transport isn’t possible. Pfizer and BioNTech have already worked on and increased the stability of mRNA vaccines.
From rock star dreams to scientific stardom
This brings us to a fascinating conversation I recently had with Professor Jeremiah A. Johnson — a professor at the MIT Department of Chemistry. Long before his time of working at a lab bench, Professor Johnson dreamed of a very different kind of stardom.
Growing up, Johnson imagined himself as a rock star or a major league baseball player. When he enrolled at Washington University in St. Louis, it was largely because the baseball coach promised him a spot on the starting lineup. He even planned to major in music—chasing his dream of becoming a rock star while balancing baseball.
However, financial pressures led him to pursue something “practical.” Electrical engineering seemed like a safe bet, especially since it overlapped with his interest in building recording studios. Yet, after slogging through circuits and electromagnetism, Professor Johnson quickly realized this wasn’t his calling.
The accidental chemist
While aimlessly following the pre-med crowd in his dorm, Professor Johnson decided to take organic chemistry in his sophomore year—a class he initially saw as a checkbox for medical school. But what started as a whim turned into a passion.
“It’s like creating art. You’re sculpting molecules from scratch.” It was just as much of a science as an art; you are literally creating and designing molecules from scratch. Just how a sculptor makes a sculpture, he was creating a molecule and there is some kind of creativity and artistic beauty behind it.
At the same time, Professor Johnson picked up a job washing glassware in Professor Karen Wooley’s chemistry lab. He didn’t know it then, but Wooley was a pioneer in polymer chemistry and drug delivery. Over time, instead of just mindlessly washing glassware, he began asking the students at the lab about the experiments they were working on. One day, a graduate student encouraged him to join the lab as an undergraduate researcher.
By the end of his sophomore year, he declared a double major in Chemistry and Biomedical Engineering, with a minor in Music. His rock star dreams had shifted—now, he wanted to become a professor leading a groundbreaking lab, much like Wooley.
Why drug delivery?
Johnson’s journey into drug delivery stems from his love of chemical biology. But practicality also played a role. In a field driven by funding priorities, drug delivery offered an undeniably impactful application for his creative molecular designs.
If you can deliver drugs selectively to diseased cells, you can improve efficacy and reduce side effects: that combination saves lives.
His lab now focuses on creating polymer-based nanoparticles that can adapt to the unique needs of different diseases. For ovarian cancer, the challenge lies in overcoming drug resistance. For multiple myeloma, it’s about precision—ensuring that treatments don’t harm healthy tissue.
Lessons
I think Professor Johnson’s story offers a couple key takeaways:
You don’t have to have everything figured out. It’s okay to try different paths and let your interests evolve. A chance class or a part-time job could lead you to your passion. Professor Johnson often sees graduate students who don’t entirely know what they want.
Merge your passion with practicality. Professor Johnson saw chemistry through the lens of art and that made him enjoy doing it. He was able to retain the artistic process he loved but also have a translational impact.
Take initiative. Ask questions, seek opportunities, and immerse yourself in the things that excite you. Johnson’s curiosity turned a glassware-cleaning gig into a lifelong career.
Now lets get to Professor Johnson’s work.
Professor Johnson and I discussed two of his studies —published in Nature Nanotechnology (2022) and Journal of the American Chemical Society (2014)—that highlight his development of bottlebrush prodrugs, with each study adding modifications to the initial concept. Each study attacks a core problem in drug delivery and proposes a carefully engineered molecular solution.
Complexity in cancer drug delivery
Modern medicine increasingly relies on a single, deceptively simple concept: get the right drug to the right place at the right time. But this is far easier said than done — especially in cancer treatment which relies on combination chemotherapy, where multiple drugs are administered together to increase tumor cell death and prevent drug resistance. The problem is the treatment needs to be delivered in precise ratios, simultaneously, and only at the site of a tumor. Each drug behaves differently in the body: it may degrade too quickly, cause toxicity to healthy cells, or fail to reach the tumor entirely. The challenge thus lies in delivering all these drugs in the exact synergistic ratio (the ratio that works best when all drugs enhance each other’s effectiveness) directly to the tumor site.
Delivering chemotherapy drugs in nanoparticle form could help reduce side effects by allowing the drugs to release directly at the tumor site, reducing toxic side effects and improving efficacy. In recent years, scientists have developed nanoparticles that deliver one or two chemotherapy drugs, but it has been difficult to design particles that can carry any more than that in a precise ratio. Furthermore, only a handful of nanoparticle drug formulations have received FDA approval to treat cancer, and only one of these particles carries more than one drug.
Johnson’s team saw an opportunity: what if you could design a nanoparticle that is pre-loaded with multiple drugs—built from the ground up with the exact ratio and release profile you want? That’s where the bottlebrush prodrug concept came in. Instead of building the delivery vehicle and then attaching drug molecules, Professor Johnson created building blocks that already include the drug. These building blocks can be joined together in a very specific structure, and the researchers can precisely control how much of each drug is included.
The creation process
Professor Johnson’s bottlebrush prodrug concept came about after a series of reflections. He first examined traditional constructions of drug delivery vehicles:
The first popular concept is building a vehicle that will circulate throughout the body (such as nanoparticles)
These vehicles are characteristically small - less than 100 nanometers
The drug can be encapsulated, which is easier to make but you have less control of when the drug releases and it is less adaptable because you have to design the nanoparticle to accommodate the drug’s physical properties. As a result, one drug might work, but then if you try to use a different drug — with different physical properties — it might not be encapsulated within the delivery vehicle.
The drug can also be chemically bonded to a polymer chain: a polymer-drug conjugate. This offers much more programmability: control over how the drug is bonded, the drug ratio, and how/when the drug gets released. On the other hand, the chemistry to create this vehicle is incredibly complex, especially if you are trying to bond multiple drugs. At the time, there were two templates for polymer-drug conjugates
A linear polymer chain with drugs attached
A star-shaped polymer (multiple arms attached to a core). Onto the ends of that star polymer you can attach your drug molecules
In both of these approaches they have surface exposed payloads (visible to the “outside world” — our body — and readily available to be cleaved by an enzyme). This could cause an undesired release when the drugs come out too early (losing what is called selectivity: the degree to which a drug acts on a given site relative to other sites)
Another issue, similar to the encapsulation method, is that the drug’s physical properties affect the whole vehicle. If a drug is very hydrophobic (”afraid” of water) and you attach it to your polymer, even if that polymer is hydrophilic (loves water), the polymer will become hydrophobic and unable to dissolve/bond with water. Instead, it will aggregate (stick together). If you change to a different drug, it might now all of a sudden become soluble again. Again, because these drugs are present on the surface, the drugs physical properties will dominate the properties of the polymer drug conjugate.
An implant: something that sticks to the body and then releases the drug
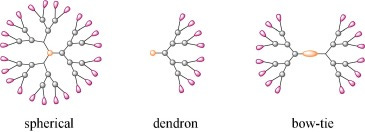
Clearly there needs to be a shift in drug delivery vehicle fabrication. Professor Johnson was inspired by a paper in 2006, outlining the design of a biodegradable bowtie dendrimer as a drug delivery vehicle of doxorubicin. A bowtie dendrimer is a highly branched polymer involving a base and then “branches” splitting off the base just like a tree, except this time it is from both ends — like in a bowtie.
In this paper, 16 of the arms had polyethylene glycol (PEG: a biocompatible polymer that shields the drug from immune detection and improves stability. PEG is found everywhere — in our shampoo, soap, and even the COVID vaccine was coated in PEG) chains and then the other 16 had drug molecules attached. The PEG chains are much longer than the core, which allow them to wrap around and shield the core and the chains with drugs. As opposed to traditional drug delivery vehicles where the drug’s physical properties dictate the systems’, the overall physical properties of this large structure are basically the same because the drugs are on the inside.
Yet, this structure had its limitations:
Spherical symmetry. If you want to conjugate (bind) this to an antibody or a specific cell, it is hard to do so because there is not one specific place.
Synthesis. Making this structure is actually super difficult. In principle you could change the drug molecules to anything you want, but you'd have to go back and spend a year synthesizing this molecule every time.
The most critical point from this structure is that drugs and PEG chains can be combined in one single nanostructure and these PEG chains protect the drugs
This paper inspired Professor Johnson’s idea of a bivalent bottlebrush: the molecule has a rigid backbone with polymers on each repeat unit, extending the backbone. This molecule is similarly resistant against the drug’s physical properties, but the bonus of having drug molecules on every repeat unit is that his vehicle has cylindrical symmetry; the ends of the molecules provide a place to conjugate to an antibody.
This molecule is also far easier to synthesize, at least in principle, because rather than loading the drugs into a pre-made particle, the vehicle could be created using modular building blocks — each one a combination of:
A drug molecule
A linker (chemical bond that can be cleaved to release the drug)
A PEG chain. PEG chains hide the drug from the body’s enzymes, delaying release until the nanoparticle reaches the target site.
Hundreds of these blocks were then linked together using a method Johnson’s lab pioneered called brush-first polymerization. Unlike older methods that try to attach drugs to a finished polymer, this process starts with monomers— the building blocks— and are then linked into a full bottlebrush polymer in a single, controlled step. The bottlebrush shape looks like a central linear backbone with densely packed side chains extending outward—like bristles sticking out from a brush. In Professor Johnson’s design, each of these bristles is a prodrug: a drug that’s chemically inactivated and reactivated only when it reaches the right biological conditions.
This synthesis strategy is revolutionary because:
It avoids complicated post-synthetic modifications. The polymer can be built in a single step once the building blocks are constructed.
It ensures consistency across batches.
It allows researchers to mix and match drugs by simply mixing the corresponding monomers in the desired ratio.
Another significant advantage of the bottlebrush prodrug platform is instead of measuring the synergy (how effective the drugs are together) with drugs in a lab dish, Professor Johnson’s team tested drug combinations within the nanoparticle structure itself, giving a far more realistic view of how the drugs will behave in the body. Traditionally, researches test potential drug combinations by exposing cancer cells in a lab dish to different concentrations of multiple drugs, yet these findings often don’t translate to the human body. Testing the drugs while already embedded gives a more realistic view of how they’ll behave.
Theoretically, this system could be scaled for more drugs. If you wanted five drugs, you could make five distinct building blocks, each with a different drug in side, and assemble them into a particle. In principle, there’s no limitation on how many drugs you can add, and the ratio of drugs carried by the particles just depends on how they are mixed together in the beginning.
This new system essentially removes all the guesswork of wondering how different drugs will affect the overall nanoparticle or whether the physical properties of the drugs are compatible with the vehicle’s
Study 1: a new era of triple-drug delivery for ovarian cancer
In the 2014 study published in Journal of the American Chemical Society, Johnson’s lab tackled a long-standing issue in cancer nanomedicine: how to load multiple chemotherapeutic drugs into a single delivery vehicle while controlling their dosage and release.
Goal:
To create a new type of nanoparticle capable of carrying three common ovarian cancer drugs—cisplatin, doxorubicin, and camptothecin—in precise ratios, with each drug released through a different mechanism, and all protected from premature degradation.
Professor Johnson showcased his bottlebrush prodrug carrying three different cancer drugs each with a different release mechanism:
Cisplatin: Released upon exposure to glutathione, an antioxidant present inside cells.
Camptothecin: Released by esterases, enzymes also common inside cells.
Doxorubicin: Doxorubicin was attached using a photo-cleaver linker, so that when ultraviolet (UV) light is emitted, it has enough intensity that the linker absorbs the light and releases the drug. This was mainly a proof of concept to show that external stimuli can also trigger a release. The biggest limitation is that light doesn’t go through your body so the tumor has to be near the surface and it would probably require creating a hole.
Once all three drugs are released, all that is left behind is PEG, which is easily biodegradable. This kind of control means that drugs can be timed to act in succession, a strategy that can enhance therapeutic effects and reduce side effects.
Impact:
Johnson’s triple-drug nanoparticle showed higher cancer cell kill rates than any single- or dual-drug particles. Moreover, the approach provided a scalable path to tailor nanoparticles for any combination of drugs. It laid the foundation for applying this technology to other types of cancer.
Study 2: tackling multiple myeloma with precision nanomedicine
In this more recent and expansive 2022 study, Johnson’s team pushed their bottlebrush system further—this time focusing on multiple myeloma, a cancer of plasma cells found in bone marrow. Healthy plasma cells help fight infections by making proteins called antibodies, which find and attack germs. In multiple myeloma, cancerous plasma cells build up in bone marrow — the soft matter inside bones where blood cells are made. In the bone marrow, the cancer cells crowd out healthy blood cells. Rather than make helpful antibodies, the cancer cells make proteins that don't work right. This leads to complications of multiple myeloma.
The challenge:
Multiple myeloma is usually treated with a three-drug combination: bortezomib (a proteasome inhibitor), pomalidomide (an immune modulator), and dexamethasone (an anti-inflammatory). But giving these drugs separately often leads to poor targeting, side effects, and non-synergistic absorption—meaning the drugs don’t reach the tumor in the effective ratio.
Using their bottlebrush prodrug platform, Johnson’s lab created nanoparticles that carry all three drugs at once. Using these particles, Professor Johnson was able to calculate and then deliver the optimal ratio of three cancer drugs used to treat multiple myeloma.
Experimental Design:
Mice with multiple myeloma tumors were treated with:
Free drugs in solution. The researchers showed that nanoparticles carrying three drugs in the synergistic ratio identified tumors much more than when the three drugs were given at the same ratio but untethered to a particle.
Mixtures of three single-drug nanoparticles
One three-drug bottlebrush nanoparticle
Interestingly, they also tested a bortezomib-only bottlebrush. Bortezomib is a proteasome inhibitor, a type of drug that prevents cancer cells from breaking down the excess proteins they produce. Accumulation of these proteins eventually causes the tumor cells to die. When bortezomib is given on its own, the drug tends accumulate in red blood cells, which have high proteasome concentrations (not ideal). However, when the researchers gave the bottlebrush prodrug version of the drug, they found that the particles accumulated primarily in plasma cells because the bottlebrush structure protects the drug from being released right away, allowing it to circulate long enough to reach its target. This shows that the structure improved efficacy with even just one drug by improving its bioavailability.
Result: The three-drug bottlebrush significantly outperformed all other groups in reducing tumor size.
The versatility of this nanoparticle platform, as demonstrated by these two studies, means it could potentially be deployed to deliver drug combinations against a variety of cancers, because you are able to apply such a variety of combinations of drugs.
Conclusion
Professor Johnson’s work embodies the best of scientific creativity and engineering pragmatism. His bottlebrush prodrug platform doesn’t just solve one problem—it offers a new way of thinking about drug design, delivery, and disease treatment. And this revolutionary work came from someone who didn’t even plan on being a scientist; life truly has an incredible way of surprising us.